16+ Chapter 9 Review Stoichiometry Section 2
Solutions are all around us. Once you join your AP class section online youll be able to access AP Daily videos any assignments from your teacher and your assignment results in AP Classroom.
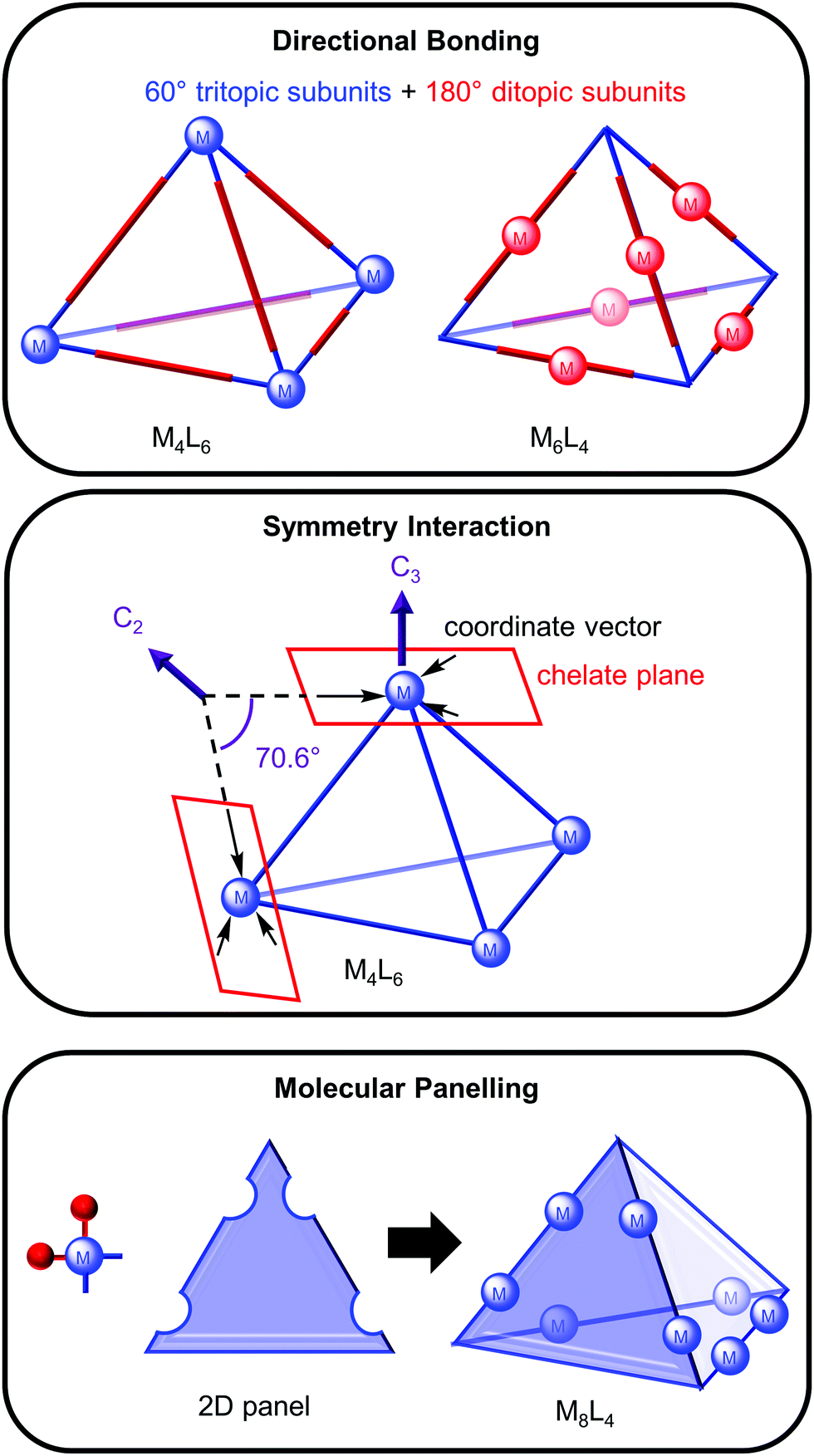
Metallosupramolecular Cages From Design Principles And Characterisation Techniques To Applications Chemical Society Reviews Rsc Publishing Doi 10 1039 D1cs01143j
163 Electrode and Cell Potentials.

. In this case however masses not molar amounts are provided and requested so additional steps of. Define three common types of chemical reactions precipitation acid-base and. Although alchemy made some useful contributions to how to manipulate matter it was not scientific by modern standards.
Web CONNECT WITH US. 186 Occurrence Preparation and Properties of Carbonates. 184 Structure and General Properties of the Nonmetals.
To discuss the relationship between the concentration of a solution and the resulting number of ions the term equivalents is used. Web When 1 mole of NH 4 2 Cr 2 O 7 is dissolved it results in 3 moles of ions 1 mol of Cr 2 O 7 2 anions and 2 mol of NH 4 cations within the solution Figure 811. Air for example is a solution.
Web The SI unit of pressure is the pascal Pa with 1 Pa 1 Nm 2 where N is the newton a unit of force defined as 1 kg ms 2One pascal is a small pressure. 173 Electrode and Cell Potentials. Air for example is a solution.
If you live near a lake a river or an ocean that body of water is not pure H 2 O but most probably a solution. Web where the concentrations are those at equilibrium. Web A nitrogen atom has seven electrons.
Web Remember that a number raised to the zero power is equal to 1 thus CO 0 1 which is why the CO concentration term may be omitted from the rate law. Round to three significant digits. Be sure to take both stoichiometry and limiting reactants into account when determining the.
Web Solution The approach used previously in Example 48 and Example 49 is likewise used here. The rate of reaction is solely dependent on the concentration of NO 2A later chapter section on reaction mechanisms will explain how a reactants concentration can have no effect on a reaction. Norton Company Inc.
If you live near a lake a river or an ocean that body of water is not pure H 2 O but most probably a solution. HCl a q NaOH. The equation becomes L T 2 g4π 2.
Types of chemical reactions. 185 Occurrence Preparation and Compounds of Hydrogen. Web 171 Review of Redox Chemistry.
That is we must derive an appropriate stoichiometric factor from the balanced chemical equation and use it to relate the amounts of the two substances of interest. Aq to form 00500 mol of NaClaq 29 kJ of heat are produced. 174 Potential Free Energy.
Molecular and Ionic Compound Structure and Properties. In the ground state they are arranged in the electron configuration 1s 2 2s 2 2p 1 x 2p 1 y 2p 1 zIt therefore has five valence electrons in the 2s and 2p orbitals three of which the p-electrons are unpaired. Web 171 Review of Redox Chemistry.
The length comes out to be 024824 m. Web Join an activity with your class and find or create your own quizzes and flashcards. Write a balanced thermochemical equation for the reaction of one mole of HCl.
Web Oxygen is the chemical element with the symbol O and atomic number 8. Contact. 79 of exam score.
It is a member of the chalcogen group in the periodic table a highly reactive nonmetal and an oxidizing agent that readily forms oxides with most elements as well as with other compoundsOxygen is Earths most abundant element and after hydrogen and helium it is the third-most. 1200 AM to 900 PM EST Saturday. B While the equipment used by Alma Levant Hayden in this 1952 picture might not seem as sleek as you might find in a lab today her approach was highly methodical.
Social Media Directory. Diversity Equity. As was previously demonstrated in this chapters section on entropy the spontaneity of a process may depend upon the temperature of the system.
Recall from Chapter 1 that solutions are defined as homogeneous mixtures that are mixed so thoroughly that neither component can be observed independently of the other. By the end of this section you will be able to. Web Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more.
Calculating Free Energy Change for a Coupled Reaction. 173 Electrode and Cell Potentials. 174 Potential Free Energy.
Recall from Chapter 1 that solutions are defined as homogeneous mixtures that are mixed so thoroughly that neither component can be observed independently of the other. It has one of the highest electronegativities among the elements 304 on the Pauling scale exceeded only by. Web United Nations Sustainable Development Goals - Time for Global Action for People and Planet.
The latter amount is most convenient and would simply involve the use of molar masses instead of atomic and formula masses as demonstrated Example 310As long as the molecular or empirical formula of the compound in question is. 183 Structure and General Properties of the Metalloids. Web Figure 12 a This portrayal shows an alchemists workshop circa 1580.
164 Potential Free Energy. The larger the K a of an acid the larger the concentration of H 3 O H 3 O and A relative to the concentration of the nonionized acid HA in an equilibrium mixture and the stronger the. Insert 100 s in for T and 98 ms 2 in for g.
182 Occurrence and Preparation of the Representative Metals. In many cases it is more convenient to use units of kilopascal 1 kPa 1000 Pa or bar 1 bar 100000 Pa. In the United States pressure is often measured in pounds of force on an area of one square.
Web This same approach may be taken considering a pair of molecules a dozen molecules or a mole of molecules etc. The probability of finding all particles in only one box either the left box or right box is then 1 16 1 16 2 16 1 16 1 16 2 16 or 1 8. Modification of work by the Italian voiceFlickr.
Square both sides of the equation to remove the radical. Although water is a reactant in the reaction it is the solvent as well so we do not include H 2 O in the equation. Give attention to your algebra.
Solutions are all around us. Web The least probable configuration of the system is one in which all four particles are in one box corresponding to distributions a and e each with a probability of 1 16. By the end of this section you will be able to.
Web 161 Review of Redox Chemistry. Define three common types of chemical reactions precipitation acid-base. Web Figure 717 The molecular structure of the methane molecule CH 4 is shown with a tetrahedral arrangement of the hydrogen atomsVSEPR structures like this one are often drawn using the wedge and dash notation in which solid lines represent bonds in the plane of the page solid wedges represent bonds coming up out of the plane and dashed lines.
Web Use the equation T 2πLg5. 1000 AM to 800 PM EST. Modification of work by vxlaFlickr.

Chapter 9 Review

Chapter 9 Stoichiometry Pages Intro To Stoichiometry All Stoichiometric Calculations Start With A To Solve You Ppt Download

Interfacial Unit Dependent Catalytic Activity For Co Oxidation Over Cerium Oxysulfate Cluster Assemblies Acs Applied Materials Interfaces

Grids With Unusual High Nuclearity A Structural Approach Stadler 2009 European Journal Of Inorganic Chemistry Wiley Online Library

Diastereoselective Control Of Tetraphenylethene Reactivity By Metal Template Self Assembly Kennedy 2019 Chemistry A European Journal Wiley Online Library

Pdf Cellulosic Substrates For Removal Of Pollutants From Aqueous Systems A Review 1 Metals Liliana Cerasella Indolean Academia Edu

The Equilibrium Constant Kc Part 2 Teaching Resources

Focus Tg4 Kssm Chemistry Terbitan Penerbitan Pelangi Sdn Bhd Flip Ebook Pages 1 48 Anyflip

Historical Development Of Al30 Highlighting The Unique Characteristics And Application In Water Treatment A Review Sciencedirect

Pdf Alternative Conception Nia Aprillia Academia Edu

09 Answer Key Pdf

Kami Export Chapter 9 Study Guide Pdf P 4 Alex Walcott Name Class Date 5 6 20 Chapter 9 Review Stoichiometry Section 1 Short Answer Answer Course Hero

Ch 9 Review

Chemestry Misconceptions Update Tcm18 188603

Complex Formation Of Niii Cuii Pdii And Coiii With 1 2 3 4 Tetraaminobutane Zimmer 2001 Chemistry A European Journal Wiley Online Library

Kami Export Chapter 9 Study Guide Pdf P 4 Alex Walcott Name Class Date 5 6 20 Chapter 9 Review Stoichiometry Section 1 Short Answer Answer Course Hero

Blog Archives Ms Mclarty S Classes